 作者
作者  通讯作者
通讯作者
《分子植物育种》网络版, 2013 年, 第 11 卷, 第 29 篇 doi: 10.5376/mpb.cn.2013.11.0029
收稿日期: 2013年08月10日 接受日期: 2013年08月13日 发表日期: 2013年11月12日
引用格式(中文):
张士云, 2013, 生长素早期响应基因Aux/IAA的研究进展, 分子植物育种(online), 11(29): 1211-1218 (doi: 10.5376/mpb.cn.2013.11.0029)
引用格式(英文):
Zhang S.Y., 2013, Progress in the Early Auxin Response Gene Aux/IAA, Fenzi Zhiwu Yuzhong (online) (Molecular Plant Breeding), 11(29): 1211-1218 (doi: 10.5376/mpb.cn.2013.11.0029)
Aux/IAA (auxin/indole-3-acetic acid)作为生长素早期响应基因,其蛋白产物能够特异性结合生长素响应因子(auxin response factor, ARF),进而调控生长素响应基因的表达,在整个植物生长素信号转导过程中具有重要作用。本文全面阐述了Aux/IAA基因的结构、作用机制及在不同植物中的功能等方面的研究进展。随着不同植物中Aux/IAA基因家族的克隆,其生理功能和对植物生长发育的调控机制将更加清晰,从而为从基因水平实现物种改良奠定基础。
生长素作为最早发现并且一直被广泛研究的一类植物激素,在生长发育众多阶段都发挥重要作用,包括影响植株胚胎发生、根系发生和伸长、维管组织分化、侧枝和花器官形成、果实发育、顶端优势和向性运动等(Hagen and Guilfoyle, 2002; Friml, 2003)。生长素之所以能够对植物产生如此大的影响,是由于它不仅可以直接作用于细胞膜而引起细胞的快速反应,而且还能够在分子水平上特异性地调控基因表达(Theologis, 1986; Guilfoyle et al., 1998)。在生长素信号传导过程中, 至少有Aux/IAA (auxin/indole-3-acetic acid)、SAUR (small auxin-up RNA)与GH3 (Gretchen Hagen 3)三类基因家族能够在极短的时间内响应生长素诱导,这些基因即被称为生长素早期响应基因(Guilfoyle et al., 1998)。其中Aux/IAA基因的蛋白产物及其作用机理是目前为止研究的较为清楚的。本文综合中国国内外最新研究成果,从Aux/IAA基因结构、作用机制及在植物中研究进展进行全面介绍,以便深入挖掘其应用价值,并为物种改良奠定理论基础。
1 Aux/IAA基因的结构特征
1982 年Walker J.C.和 Key J.L.首次在大豆上分离得到Aux/IAA基因,并将其称为能够快速响应生长素诱导的一类基因。之后在豌豆(Yamamoto et al., 1992)、拟南芥(Abel and Theologis, 1995)、烟草(Dargeviciute et al., 1998)、棉花(Suo et al., 2004)、番茄(Wang et al., 2005)、马铃薯(Kloosterman et al., 2006)、杨树(董秀春等, 2007)、水稻(Song et al., 2009)、草莓(Liu et al., 2011)和苹果(Devoghalaere et al., 2012)等植物中也克隆到了该基因,并进行了相关的研究。但在细菌、动物和真菌中尚没有发现这类基因,因此可能为植物所特有。
典型的Aux/IAA蛋白具有四个保守结构域,分别称为Domain I、II、III和IV,如图1所示。Domain I、II位于氨基端(N端),而Domain III和IV位于羧基端(C端)。研究表明,四个保守的结构域都具有一定的功能,Domains I具有一个与乙烯响应因子相关的两亲性基序LXLXLX,能够与共阻遏物结合,是Aux/IAA蛋白的转录抑制功能所必需的区域(Tiwari et al., 2001; 2004; Szemenyei et al., 2008)。Domains II是Aux/IAA蛋白被泛素化降解的靶位点,其核心序列为VGWPP,该区域的显性突变会使Aux/IAA蛋白不能够进入泛素化途径而导致稳定性增强(Zenser et al., 2001, 2003; Kepinski and Leyser, 2004; 2005)。Domains III和IV是与生长素响应基因转录因子ARF (auxin response factor)结合的部位,其二级结构能够折叠成一个螺旋-转角-螺旋(βαα),合成的多肽能够在体外进行折叠和二聚化(Morgan et al., 1999)。Domains IV也可能有助于二聚化。在Domains II和Domains IV一般还存在两个核定位信号NLS (nuclear localization signal) (Kim et al., 1997)。此外,在Domain I和II之间还有一个光敏色素A的磷酸化作用位点,因此推测Aux/IAA蛋白可以通过光敏色素A的磷酸化作用介导生长素与光信号通路(Colón-Carmona et al., 2000)。
2 Aux/IAA 基因的作用机制
生长素信号转导包括信号感知、生长素响应基因的表达以及最终在特定植物组织部位表现出相应的生理反应等一系列过程(Guilfoyle et al., 1998; Santner and Estelle, 2009; Chapman and Estelle, 2009)。近几年来,关于生长素信号转导的研究已经取得突破性进展,有关生长素信号转导中各个元件的功能和作用也有了更加深入的认识。
其中,细胞内响应生长素转录的主要有两个大的转录因子家族,分别是Aux/IAA蛋白家族和生长素转录因子ARF。ARF是一类具有DNA结合域的转录因子,是调节生长素应答反应,控制基因表达的直接分子。它可以结合到特定基因启动子区域的生长素反应元件AuxRE (auxin response element)而激活或者抑制生长素响应基因的表达(Ulmasov et al., 1999)。迄今为止,在拟南芥中共发现有23个ARFs,其中大多数促进生长素响应基因的表达,也有一些抑制基因表达,这主要取决于ARF蛋白中间保守域的特性(Tiwari et al., 2003)。研究表明,ARF的C末端结构域CTD (C-terminal domain)与Aux/IAA蛋白的Domains III和IV高度同源,二者通过这两个区域形成二聚体,进而调控生长素响应基因的转录(Tiwari et al., 2003)。由此可见,生长素早期响应基因在整个生长素信号转导通路中发挥重要作用。
Aux/IAA基因编码一种18-35 kD的短命蛋白,大部分Aux/IAA蛋白的半衰期极短(Reed, 2001),他们的降解依赖于生长素及其细胞内的受体TIR1 (transport inhibitor response1)。研究表明,TIR1是一类F-box蛋白家族,其与拟南芥中 Skp1蛋白质类似物ASK1和ASK2及Cullin (拟南芥中称为AtCul1)可以形成 SCFTIR1 复合体(Gray et al., 2001)。SCF复合体是一种非常重要的E3泛素连接酶,生长素就是通过SCFTIR1介导的泛素—蛋白酶体系统来实现对Aux/IAA蛋白的降解(Dharmasiri et al., 2005)。
研究表明,Aux/IAA蛋白的快速降解对于生长素的信号转导过程是必需的(Worley et al., 2000),生长素的存在可以促进Aux/IAA蛋白的降解。对TIR1的结构研究揭示,位于TIR1 LRR (leucine-rich-repeats)区域上的一个疏水腔既能结合生长素,又能结合Aux/IAA Domain II (Tan et al., 2007)。当细胞内生长素浓度较低时;Aux/IAA蛋白通过与ARF结合而抑制ARF对下游生长素响应基因的调控;当细胞内生长素浓度较高时,生长素通过其侧链的梭基端与TIRI分子基部的LRR结合,进而促进Aux/IAA蛋白的Domains II直接结合到生长素的上方,形成SCFTIR1-Auxin-Aux/lAA复合体。处于该复合体中的Aux/IAA蛋白在泛素活化酶E1、泛素结合酶E2及泛素连接酶E3酶的作用下被蛋白质列解体所降解。Aux/IAA蛋白对ARF的抑制作用随之解除,使其重新启动对下游基因(包括生长素早期响应基因)的调控(Tan et al., 2007),如图2所示。
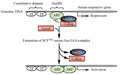 图2 细胞内的生长素信号传导过程 (Chapman and Estellem, 2009) Figure 2 Process of Auxin intracellular signal transduction (Chapman and Estelle, 2009) |
早期研究表明Domain I在Aux/IAA的转录抑制过程中是必需的,但是其作用机制并不十分清楚(Tiwari et al., 2004)。现在已经发现一些蛋白质如PICKLE和TOPLESS分别与拟南芥Aux/IAA蛋白SLR/IAA14和BDL/IAA12的Domain I结合,然后作为共阻遏物Corepressor (CoRep)抑制ARF的转录功能(Fukaki et al., 2006; Szemenyei et al., 2008)。但是,由于每个相关的蛋白都是一个大家族(TIR1/AFBs 6个, Aux/IAAs 29个, ARFs 23个, TPL/TOPLESS 5个),对于不同家族成员之间相互作用的特异性仍需深入研究。
3 Aux/IAA基因的功能
Aux/IAA基因属于多基因家族。目前在拟南芥中已发现有29个成员(Liscum and Reed, 2002),在水稻和玉米中均有31个成员(Song et al., 2009; Wang et al., 2010a),杨树中有35个成员(Kalluri et al., 2007),高粱、番茄和草莓中有26个(Audran-Delalande et al., 2012; Devoghalaere et al., 2012; Wang et al., 2010b),棉花中有10个(索金凤等, 2004; 韩晓勇, 2010),黄瓜中有28个(王垒等, 2011),马铃薯中有27个(Wu et al., 2012),苹果中40个(Devoghalaere et al., 2012)。
目前,对Aux/IAA基因功能的研究主要是通过拟南芥功能获得突变体,它们都是Aux/IAA蛋白Domain II核心区(VGWPP)的某个氨基酸发生了改变,导致突变体产生诸多生长素相关的异常表型,包括影响根和下胚轴的发育和向光性,以及植株和叶片的形态建成。例如AXR2/IAA7的显性突变体axr2 (auxin resistance 2)出现严重的矮化、根的向地性和茎的背地性缺失、下胚轴变短和无根毛,进一步研究表明该突变体下胚轴和花薹的表皮细胞的长度变短、细胞数目减少(Timpte et al., 1992; 1994);通过对axr2-1突变体进行诱变处理,得到AXR2/IAA7的两个功能缺失突变体axr2-1-r3和axr2-1r4,它们分别为AXR2/IAA7蛋白Domain III和Domain I的某个氨基酸发生改变,二者表型相似并且介于突变体axr2-1与野生型之间(Nagpal et al., 2000);SHY2/IAA3的突变体shy2 (short hypocotyl 2)的下胚轴变短、侧根减少、根向地性减弱、叶片向上卷曲,并且黑暗条件下能够形成叶片(Kim et al., 1998; Tian and Reed, 1999; Tian et al., 2002);SLR/IAA14的突变体slr-1 (solitary root 1)没有侧根,根毛较少,根与下胚轴的向地性异常(Fukaki et al., 2002);AXR5/IAA1突变体axr5-1侧根减少,根、下胚轴向地性和茎的向光性减弱,植株顶端优势丧失,莲座叶变小,种子数量减少等,突变体中检测到多种生长素早期响应基因表达水平降低;构建axr5-1tir1-1双突变体,抗生长素表型比axr5-1单突变体更加明显,表明Aux/IAA蛋白和TIR1各自影响生长素的响应;MSG2/IAA19的突变体msg2的下胚轴完全失去向地性(Tatematsu et al., 1999);IAA28突变体iaa28-1侧根减少,花薹变短,此外,IAA28基因具有高度的组织表达特异性,只在根和花薹中显著表达,与突变体表型相一致;AXR3/IAA17的半显性突变体axr3-1根系变短,侧根增多,顶端优势增强(Leyser et al., 1996)。拟南芥突变体研究表明,Aux/IAA基因的功能具有特异性,在植株不同组织部位对生长素的响应不同,既可能有促进作用,也可能具有抑制作用;并且大部分突变体产生诸多抗生长素的表型,如侧根减少、根的向地性减弱、顶端优势丧失、叶片变小及种子减少等,这与对应Aux/IAA蛋白稳定性增强而导致对生长素响应减弱相一致;个别突变体则出现一些对生长素敏感的表型,如侧根增多,顶端优势增强等,这可能与不同Aux/IAA基因所对应的ARF的目标生长素响应基因及调控方式不同有关。此外,有些突变体具有相同或者相近的表型,表明Aux/IAA基因还具有重叠性,许多Aux/IAA基因的功能缺失突变体并没有表现出明显的突变表型,也可能是由于大的基因家族内基因功能冗余导致(Parry and Estelle, 2006)。
不同植物中Aux/IAA基因的克隆和转基因研究也取得了一定进展。OsIAA1是从水稻中分离到的生长素早期响应基因,用生长素处理后,胚芽鞘中OsIAA1表达水平能在短时间内显著增加,而外源生长素消耗后表达量又很快下降,超表达OsIAA1的转基因植株对生长素敏感性减弱,植株矮化、株型松散,并导致主根数目,长度增加,侧根增多(Song et al., 2009);而超表达OsIAA1 Domain II突变体的植株下胚轴和茎伸长及叶片增大,细胞解剖研究表明转基因植株的花序和叶片细胞长度变短、数目减少,表明IAA1可能参与水稻地上部分细胞伸长和分化(Ku et al., 2009);超表达OsIAA3 Domain II突变体导致转基因植株对生长素的敏感性减弱,向地性消失,侧根和不定根变短且数目减少,影响叶片和维管组织的发育(Nakamura et al., 2006),研究发现,在突变体OsIAA3中依赖生长素诱导表达的不定根形成相关基因CRL1的表达被抑制,而CRL1编码一类LOB/ASL转录因子,因此推测生长素通过Aux/IAA与ARF相互作用调节LOB/ASL,进而调控不定根的发育;马铃薯StIAA2 基因下调导致株高增加、叶柄下偏和顶端叶原基极度弯曲等表型(Kloosterman et al., 2006),表明StIAA2对植株地上部分的发育有重要作用;番茄SlIAA9基因下调导致单叶和单性结实,表明SlIAA9在西红柿的叶片形态建成和坐果中具有重要作用(Wang et al., 2005);超量表达SlIAA3造成根系的向地性发生改变(Zhang et al., 2007),而反义抑制SlIAA3表达不仅导致顶端优势减弱和对生长素敏感性降低,还产生了乙烯响应相关表型(Chaabouni et al., 2009);过表达全部或部分缺失Domain II的三个拟南芥Aux/IAA 基因同样产生严重的生长素异常表型(Sato and Yamamoto, 2008),9个缺少Domain I和 II的茄属Aux/IAA基因以及一个缺少Domain II的水稻OsIAA8均能够响应生长素处理(Song et al., 2009; Wu et al., 2012),推测这些缺少保守结构域的Aux/IAA基因也可能参与生长素的信号转导途径(Wu et al., 2012);转基因研究直接揭示了Aux/IAA基因在植物生长发育过程中所发挥广泛作用,同时表明Aux/IAA基因介导的生长素信号转导途径对植物生长发育的调控是一个极为复杂的过程。
Aux/IAA基因除了响应生长素调节,同时还受其他激素、非生物胁迫以及光信号转导等影响。例如,拟南芥突变体axr2除了具有生长素抗性之外,对乙烯和脱落酸也有抗性(Allison et al., 1990);拟南芥突变体axr3-1/iaa17 能同时对生长素和乙烯产生抗性,并且引起细胞分裂素的异常反应(Leyser et al., 1996);slr-1/iaa14对生长素有抗性,但是对脱落酸敏感;iaa28对生长素、细胞分裂素和乙烯均有抗性(Rogg et al., 2001);此外,外源油菜素内酯(BR)能显著诱导AXR3/IAA17、AXR2/IAA7、SLR/IAA14和IAA2等基因的表达,在BR信号转导功能突变体bri1 和det2 植株中,AXR3/IAA17 基因的表达均减少(Kim et al., 2006);在水稻31个Aux/IAA家族成员中,大部分基因在用植物激素ABA、KN、GA、JA、IAA和BR等非生物胁迫处理后至少响应其中一种激素;许多Aux.IAA基因还能对非生物胁迫(干旱和盐)做出响应,表明它们还参与逆境的应答(Song et al., 2009)。另外,拟南芥突变体msg2/iaa19下胚轴具有反趋光性(Tatematsu et al., 1999);shy2/iaa3, axr2-1/iaa7 and axr3-1/iaa17能够在黑暗条件下产生叶片,甚至抽薹开花(Kim et al., 1996; Kim et al., 1998; Reed et al., 1998; Nagpal et al., 2000);在shy2-1幼苗中还检测到了光系统调节基因CAB和PSBA等的表达(Tian et al., 2002);光照和黑暗条件下OsIAA1具有不同的表达模式(Ku et al., 2009),表明某些Aux/IAA基因还参与光信号通路;王益军等分析高粱Aux/IAA基因的启动子区域发现除了生长素响应元件AuxRE,还存在许多与光信号转导和非生物胁迫相关的顺式调控元件,这也为Aux/IAA基因参与生长素信号与其他信号的交叉途径提供了证据(王益军等, 2010)。
4 Aux/IAA基因的研究展望
生长素的作用及其分子机制已有上百年的研究,Aux/IAA作为生长素信号转导中调节基因表达的核心因子,其分子功能的研究对于解析整个生长素信号转导过程至关重要。由于目前所揭示的Aux/IAA的功能大多是通过突变体研究得来的,因此,关于每个基因在植物生长发育过程中的确切作用还不是很清楚。ARF-Aux/IAA-CoRep之间的互作模式和ARF所调控的生长素响应基因的特异性,以及各组分的相对丰度、稳定性和对生长素的敏感性赋予了该复合体调控基因表达方式的多样性。同时,生长素信号途径与其他信号途径的交叉作用,也使得生长素信号转导通路的研究更具挑战性。今后对Aux/IAA基因功能的研究需要综合运用最新的基因组学和计算机科学研究手段,随着不同植物中Aux/IAA家族及其目标基因的克隆和研究,其生理功能及其所参与的生长素调控机制将更加清晰。这将会为进一步的基因遗传转化和物种改良奠定了基础,并为其他的激素的研究提供思路。
作者贡献
张士云是项目的构思者和负责人,独立完成了数据分析,论文写作与修改。
致谢
本研究由山东省临沂市农技推广补助项目资助。感谢山东省临沂市兰山区农业局及山东省临沂市义堂镇农业技术推广站对本研究给予的支持与帮助。
参考文献
Abel S., and Theologis A., 1996, Early genes and auxin action, Plant Physiol., 111(1): 9-17
http://dx.doi.org/10.1104/pp.111.1.9
PMid:8685277 PMCid:PMC157808
Allison M.J., Hammond A.C., and Jones R.J., 1990, Detection of ruminal bacteria that degrade toxic dihydroxypyridine compounds produced from mimosine, Appl. Environ. Microbiol., 56(3): 590-594
PMid:2317038 PMCid:PMC183391
Audran-Delalande C., Bassa C., Mila I., Regad F., Zouine M., and Bouzayen M., 2012, Genome-wide identification, functional analysis and expression profiling of the Aux/IAA gene family in tomato, Plant Cell Physiol., 53(4): 659-672
http://dx.doi.org/10.1093/pcp/pcs022
PMid:22368074
Chaabouni S., Jones B., Delalande C., Wang H., Li Z.G., Mila I., Frasse P., Latché A., Pech J.C., and Bouzayen M., 2009, Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth, J. Exp. Bo., 60(4): 1349-1362
http://dx.doi.org/10.1093/jxb/erp009
PMCid:PMC2657550
Chapman E.J., and Estelle M., 2009, Mechanism of Auxin-regulated gene expression in plant, Annu. Rev. Genet., 43: 265-285
http://dx.doi.org/10.1146/annurev-genet-102108-134148
PMid:19686081
Colón-Carmona A., Chen D.L., Yeh K.C., and Abel S., 2000, Aux/IAA proteins are phosphorylated by phytochrome in vitro, Plant Physiol., 124(4): 1728-1738
http://dx.doi.org/10.1104/pp.124.4.1728
PMid:11115889 PMCid:PMC59870
Dargeviciute A., Roux C., Decreux A., Sitbon F., and Perrot-Rechenmann C., 1998, Molecular cloning and expression of the early auxin-responsive Aux/IAA gene family in Nicotiana tabacum, Plant Cell Physiol., 39(10): 993-l002
http://dx.doi.org/10.1093/oxfordjournals.pcp.a029311
PMid:9871362
Devoghalaere F., Doucen T., Guitton B., Keeling J., Payne W., Ling T.J., Ross J.J., Hallett I.C., Gunaseelan K., Dayatilake G.A., Diak R., Breen K.C., Tustin D.S., Costes E., Chagné D., Schaffer R.J., and David K.M., 2012, A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control, BMC Plant Biol., 12: 7
http://dx.doi.org/10.1186/1471-2229-12-7
PMid:22243694 PMCid:PMC3398290
Dharmasiri N., Dharmasiri S., and Estelle M., 2005, The F-box protein TIR1 is an auxin receptor, Nature, 435 (7041): 441-445
http://dx.doi.org/10.1038/nature03543
PMid:15917797
Dong X.C., 2008, Isolation and functional characterization of genes encoding auxin signaling components in Populus tomentosa Carr, Thesis for M.S., Shandong Agricultral Univerisity, Supervisor: Fan J.H., pp.6-37 (董秀春, 2008, 毛白杨生长素信号转导因子基因的分离与功能的初步分析, 硕士学位论文, 山东农业大学, 导师: 樊金会, pp.6-37)
Dong X.C., Fan J.H., Yu X.N., Shu H.R., 2007, Cloning and sequencing of PtIAA1 from Populus tomentosa, a member of the Aux/IAA gene family, Fenzi Zhiwu Yuzhong (Molecular Plant Breeding), 5(4): 565-571 (董秀春, 樊金会, 于学宁, 束怀瑞, 2007, 一个属于Aux/IAA基因家族的毛白杨PtIAA1的克隆和序列分析, 分子植物育种, 5(4): 565-571)
Friml J., 2003, Auxin transport-shaping the plant, Curr. Opin. Plant Biol., 6: 7-12
http://dx.doi.org/10.1016/S1369526602000031
PMid:12495745
Fukaki H., Tameda S., Masuda H., and Tasaka M., 2002, Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis, Plant J., 29(2): 153-168
http://dx.doi.org/10.1046/j.0960-7412.2001.01201.x
PMid:11862947
Fukaki H., Taniguchi N., and Tasaka M., 2006, PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation, Plant J., 48(3): 380-389
http://dx.doi.org/10.1111/j.1365-313X.2006.02882.x
PMid:17010112
Gray W.M., Kepinski S., Rouse D., Leyser O., and Estelle M., 2001, Auxin regulates SCFTIR1-dependent degradation of Aux/IAA proteins, Nature, 414(6861): 271-276
http://dx.doi.org/10.1038/35104500
PMid:11713520
Guilfoyle T., Hagen G., Ulmasov T., and Murfett J., 1998, How does auxin turn on genes? Plant Physiol., 118(2): 341-347
http://dx.doi.org/10.1104/pp.118.2.341
PMid:9765520 PMCid:PMC1539191
Hagen G., and Guilfoyle T., 2002, Auxin-responsive gene expression: genes, promoters and regulatory factors, Plant Mol. Biol., 49(3-4): 373-385
http://dx.doi.org/10.1023/A:1015207114117
PMid:12036261
Han X.Y., 2010, Cloning and expression analyses of nine genes in Aux/IAA genes in Aux/IAA family in Gossypium Hirsutum L., Thesis for M.S., Nanjing Agricultral Univerisity, Supervisor: Guo W.Z., pp.21-55 (韩晓勇, 2010, 陆地棉Aux/IAA家族九个基因的克隆和表达分析, 硕士学位论文, 南京农业大学, 导师: 郭旺珍, pp.21-55)
Kalluri U.C., Difazio S.P., Brunner A.M., Tuskan G.A., 2007, Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa, BMC Plant Biol., 7: 59
http://dx.doi.org/10.1186/1471-2229-7-59
PMid:17986329 PMCid:PMC2174922
Kepinski S., and Leyser O., 2004, Auxin-induced SCFTIR1-Aux/IAA interaction involves stable modification of the SCFTIR1 complex, Proc. Natl. Acad. Sci. USA., 101(33): 12381-12386
http://dx.doi.org/10.1073/pnas.0402868101
PMid:15295098 PMCid:PMC514484
Kepinski S., and Leyser O., 2005, The Arabidopsis F-box protein TIR1 is an auxin receptor, Nature, 435(7041): 446-451
http://dx.doi.org/10.1038/nature03542
PMid:15917798
Kim B.C., Soh M.C., Kang B.J., Furuya M., and Nam H.G., 1996, Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2, Plant J., 9(4): 441-456
http://dx.doi.org/10.1046/j.1365-313X.1996.09040441.x
PMid:8624510
Kim B.C., Soh M.S., Hong S.F., Furuya M., and Nam H.G., 1998, Photomorphogenic development of the Arabidopsis shy2-1D mutant and its interaction with phytochromes in darkness, Plant J., 15(1): 61-68
http://dx.doi.org/10.1046/j.1365-313X.1998.00179.x
PMid:9744095
Kim H., Park P.J., Hwang H.J., Lee S.Y., Oh M.H., and Kim S.G., 2006, Brassinosteroid signals control expression of the AXR3/IAA17 gene in the cross-talk point with auxin in root development, Biosci. Biotechnol. Biochem., 70(4): 768-773
http://dx.doi.org/10.1271/bbb.70.768
PMid:16636440
Kim J., Harter K., and Theologis A., 1997, Protein–protein interactions among the Aux/IAA proteins, Proc. Natl. Acad. Sci. USA., 94(22): 11786-11791
http://dx.doi.org/10.1073/pnas.94.22.11786
PMid:9342315 PMCid:PMC23574
Kloosterman B., Visser R.G.F., and Bachem C.W.B., 2006, Isolation and characterization of a novel potato Auxin/Indole-3-Acetic Acid family member (StIAA2) that is involved in petiole hyponasty and shoot morphogenesis, Plant Physiol. Biochem., 44(11-12): 766-775
http://dx.doi.org/10.1016/j.plaphy.2006.10.026
PMid:17098436
Ku S.J., Park J.Y., Ha S.B., and Kim J., 2009, Overexpression of IAA1 with domain II mutation impairs cell elongation and cell division in inflorescences and leaves of Arabidopsis, J. Plant Physiol., 166(5): 548-553
http://dx.doi.org/10.1016/j.jplph.2008.07.006
PMid:18771815
Leyser H.M., Pickett F.B., Dharmasiri S., and Estelle M., 1996, Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter, Plant J., 10(3): 403-413
http://dx.doi.org/10.1046/j.1365-313x.1996.10030403.x
PMid:8811856
Liscum E., and Reed J.W., 2002, Genetics of Aux/IAA and ARF action in plant growth and development, Plant Mol. Biol., 49(3-4): 387-400
http://dx.doi.org/10.1023/A:1015255030047
PMid:12036262
Liu D.J., Chen J.Y., and Lu W.J., 2011, Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development, Mol. Biol. Rep., 38(2): 1187-1193
http://dx.doi.org/10.1007/s11033-010-0216-x
PMid:20563652
Morgan K.E., Zarembinski T.L., Theologis A., and Abel S., 1999, Biochemical characterization of recombinant polypeptides corresponding to the predicted beta-alpha-alpha fold in Aux/IAA proteins, FEBS Lett., 454(3): 283-287
http://dx.doi.org/10.1016/S0014-5793(99)00819-4
Nagpal P., Walker L.M., Young J.C., Sonawala A., Timpte C., Estelle M., and Reed J.W., 2000, AXR2 encodes a member of the Aux/IAA protein family, Plant Physiol., 123(2): 563-574
http://dx.doi.org/10.1104/pp.123.2.563
PMid:10859186 PMCid:PMC59024
Nakamura A., Umemura I., Gomi K., Hasegawa Y., Kitano H., Sazuka T., and Matsuoka M., 2006, Production and characterization of auxin-insensitive rice by overexpression of a mutagenized rice IAA protein, Plant J., 46(2): 297-306
http://dx.doi.org/10.1111/j.1365-313X.2006.02693.x
PMid:16623891
Parry G., and Estelle M., 2006, Auxin receptors: a new role for F-box proteins, Curr. Opin. Cell Biol., 18(2): 152-156
http://dx.doi.org/10.1016/j.ceb.2006.02.001
PMid:16488128
Reed J.W., 2001, Roles and activities of Aux/IAA proteins in Arabidopsis, Trends Plant Sci., 6(9): 420-425
http://dx.doi.org/10.1016/S1360-1385(01)02042-8
Reed R.C., Brady S.R., and Muday G.R., 1998, Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis, Plant Physiol., 118(4): 1369-1378
http://dx.doi.org/10.1104/pp.118.4.1369
PMid:9847111 PMCid:PMC34753
Rogg L.E., Lasswell J., and Bartel B., 2001, A gain-of-function mutation in IAA28 suppresses lateral root development, Plant Cell, 13(3): 465-480
http://dx.doi.org/10.2307/3871400
http://dx.doi.org/10.1105/tpc.13.3.465
PMid:11251090 PMCid:PMC135515
Santner A., and Estelle M., 2009, Recent advances and emerging trends in plant hormone signaling, Nature, 459: 1071-1078
http://dx.doi.org/10.1038/nature08122
PMid:19553990
Sato A., and Yamamoto K.T., 2008, Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis, Physiol. Plant., 133(2): 397-405
http://dx.doi.org/10.1111/j.1399-3054.2008.01055.x
PMid:18298415
Song Y., Wang L., and Xiong L.Z., 2009, Comprehensive expression profiling analysis of OsIAA gene family in developmental processes and in response to phytohormone and stress treatments, Planta, 229: 577-591
http://dx.doi.org/10.1007/s00425-008-0853-7
PMid:19034497
Suo J.F., Pu L., Liang X.E., and Xue Y.B., 2004, Identification of an endothelium-specific gene GhIAA16 in cotton (Gossypium hirsutum), Zhiwu Xuebao (Acta Botanica Sinica), 46(4): 472-479 (索金凤, 普莉, 梁小娥, 2004, 薛勇彪棉花胚珠内种皮特异基因GhIAA16的分离鉴定, 植物学报, 46(4): 472-479)
Szemenyei H., Hannon M., and Long J.A., 2008, TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis, Science, 319(5868): 1384-1386
http://dx.doi.org/10.1126/science.1151461
PMid:18258861
Tan X., Calderon-Villalobos L.I., Sharon M., Zheng C.X., Robinson C.V., Estelle M., and Zheng N., 2007, Mechanism of auxin perception by the TIR1 ubiquitin ligase, Nature, 446(7136): 640-645
http://dx.doi.org/10.1038/nature05731
PMid:17410169
Tatematsu K., Watahiki M.K., and Yamamoto K.T., 1999, Evidence for a dominant mutation of IAA19 that disrupts hypocotyl growth curvature responses and alters auxin sensitivity, In 10th International Conference on Arabidopsis Research (Melbourne, Australia), Abstract No.8-39
Theologis A., 1986, Rapid gene regulation by Auxin, Ann. Rev. Plant Physiol., 37: 407-438
http://dx.doi.org/10.1146/annurev.arplant.37.1.407
http://dx.doi.org/10.1146/annurev.pp.37.060186.002203
Tian Q., and Reed J.W., 1999, Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene, Development, 126(4): 711-721
PMid:9895319
Tian Y.H., Wang Y.J., Zhang Y., Knyazikhina Y.R., Bogaert J., and Mynenia R.B., 2002, Radiative transfer based scaling of LAI retrievals from reflectance data of different resolutions, Remote Sensing of Environment, 84(1): 143-159
http://dx.doi.org/10.1016/S0034-4257(02)00102-5
Timpte C., Wilson A.K., and Estelle M., 1994, The axr2-1 mutation of Arabidopsis thaliana is a gain-of-function mutation that disrupts an early step in auxin response, Genetics, 138(4): 1239-1249
PMid:7896103 PMCid:PMC1206260
Timpte C.S., Wilson A.K., and Estelle M., 1992, Effects of the axr2 mutation of Arabidopsis on cell shape in hypocotyl and inforescence, Planta, 188(2): 271-278
http://dx.doi.org/10.1007/BF00216824
PMid:24178265
Tiwari S.B., Hagen G., and Guilfoyle T., 2003, The roles of auxin response factor domains in auxin-responsive transcription, Plant Cell, 15(2): 533-543
http://dx.doi.org/10.1105/tpc.008417
PMid:12566590 PMCid:PMC141219
Tiwari S.B., Hagen G., and Guilfoyle T.J., 2004, Aux/IAA proteins contain a potent transcriptional repression domain, Plant Cell, 16(2): 533-543
http://dx.doi.org/10.1105/tpc.017384
PMid:14742873 PMCid:PMC341922
Tiwari S.B., Wang X.J., Hagen G., and Guifoyle T.J., 2001, Aux/IAA proteins are active repressors, and their stability and activity are modulated by auxin, Plant Cell, 13(12): 2809-2822
http://dx.doi.org/10.2307/3871536
http://dx.doi.org/10.1105/tpc.13.12.2809
PMid:11752389 PMCid:PMC139490
Ulmasov T., Hagen G., and Guilfoyle T.J., 1999, Activation and repression of transcription by auxin-responsive factors, Proc. Natl. Acad. Sci. USA., 96(10): 5844-5849
http://dx.doi.org/10.1073/pnas.96.10.5844
PMid:10318972 PMCid:PMC21948
Wang H., Jones B., Li Z., Frasse P., Delalande C., Regad F., Chaabouni S., Latché A., Pech J.C., and Bouzayen M., 2005, The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis, Plant Cell, 17(10): 2676-2692
http://dx.doi.org/10.1105/tpc.105.033415
PMid:16126837 PMCid:PMC1242265
Wang L., Lou L.N., Yan L.Y., Lou Q.F., and Chen J.F., 2011, A differential expression analysis of some Aux/IAA family genes in cucumber fruit during the early development, Nanjing Nongye Daxue Xuebao (Journal of Nanjing Agricultural University), 34(4): 13-17 (王垒, 娄丽娜, 闫立英, 娄群峰, 陈劲枫, 2011, 黄瓜果实发育早期Aux /IAA家族部分基因的差异表达分析, 南京农业大学学报, 34(4): 13-17)
Wang Y.J., Deng D.X., Bian Y.L., Lv Y.P., and Xie Q., 2010a, Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays L.), Mol. Biol. Rep., 37(8): 3991-4001
http://dx.doi.org/10.1007/s11033-010-0058-6
PMid:20232157
Wang S.K., Bai Y.H., Shen C.J., Wu Y.R., Zhang S.N., Jiang D.A., Guifoyle T.J., Chen M., and Qi Y.H., 2010b, Auxin-related gene families in abiotic stress response in Sorghum bicolor, Funct. Integr. Genomics, 10(4): 533-546
http://dx.doi.org/10.1007/s10142-010-0174-3
PMid:20499123
Wang Y.J., Lv Y.P., Xie Q., Deng D.X., and Bian Y.L., 2010, Whole-genome sequence characterization of primary auxin-responsive Aux/IAA family in sorghum, Zuowu Xuebao (Acta Agronomica Sinica), 36(4): 688-694 (王益军, 吕艳萍, 谢秦, 邓德祥, 卞云龙, 2010, 高粱全基因组生长素原初响应基因Aux/IAA的序列特征分析, 作物学报, 36(4): 688-694)
Worley C.K., Zenser N., Ramos J., Rouse D., Leyser O., Theologis A., and Callis J., 2000, Degradation of Aux/IAA proteins is essential for normal auxin signaling, Plant J., 21(6): 553-562
http://dx.doi.org/10.1046/j.1365-313x.2000.00703.x
PMid:10758506
Wu J., Peng Z., Liu S.Y., He Y.J., Cheng L., Kong F., Wang J., and Lu G., 2012, Genome-wide analysis of Aux/IAA gene family in Solanaceae species using tomato as a model, Mol. Genet. Genomics, 287(4): 295-311
http://dx.doi.org/10.1007/s00438-012-0675-y
PMid:22314799
Yamamoto T., Moerschell R.P., Wakem L.P., Ferguson D., and Sherman F., 1992, Parameters affecting the frequencies of transformation and co-transformation with synthetic oligonucleotides in yeastm, Yeast, 8(11): 935-948
http://dx.doi.org/10.1002/yea.320081104
PMid:1336288
Zenser N., Dreher K.A., Edwards S.R., and Callis J., 2003, Acceleration of Aux/IAA proteolysis is specific for auxin and independent of AXR1, Plant J., 35(3): 285-294
http://dx.doi.org/10.1046/j.1365-313X.2003.01801.x
PMid:12887580
Zenser N., Ellsmore A., Leasure C., and Callis J., 2001, Auxin modulates the degradation rate of Aux/IAA proteins, Proc. Natl. Acad. Sci. USA., 98(20): 11795-11800
http://dx.doi.org/10.1073/pnas.211312798
PMid:11573012 PMCid:PMC58810
Zhang J., Chen R., Xiao J., Zou L., Li H., Quyang B., and Ye Z., 2007, Isolation and characterization of SlIAA3, an Aux/IAA gene from tomato, DNA Seq., 18(6): 407-414
http://dx.doi.org/10.1080/10425170701517820
PMid:17676470